

Our people are extraordinary.
Arts & Sciences has over 1,000 faculty and staff who utilize their diverse expertise in the pursuit of research breakthroughs, gaining a deeper understanding of the world's most pressing issues and serving as mentors of the next generation.
select honors from our faculty


search for faculty:


William Acree

Rachel Adams

Cassie Adcock

Ama Bemma Adwetewa-Badu

Nazmul Ahsan

Jami Ake

W. Mark Akin

Deniz Aksoy

Mark G. Alford

Tazeen Ali

Kari Allen

Megan Allen

Elizabeth Allen

Sachiko Amari

Felix Ampadu

Jeffrey Anderson

Kenneth (Andy) Andrews

Gaetano Antinolfi

Nicola Aravecchia

Jennifer Arch

Raymond E. Arvidson

G'Ra Asim

Costas Azariadis
Recent Faculty Grants & Awards
Taylor Carlson, assistant professor of political science in Arts & Sciences, has been awarded a Social Science Research Council Social Data Research Fellowship to study the extent to which user-generated content (i.e. comments) on social media platforms distorts information reported by mainstream news outlets. The fellowship comes with a $50,000 award.
See what our faculty are working on now
More from The Ampersand
Acree awarded entrepreneurial leadership, innovation fellowship
William Acree, vice dean of interdisciplinary initiatives and innovation and a professor of Spanish, has been named a fellow with the Academy for Innovative Higher Education Leadership.
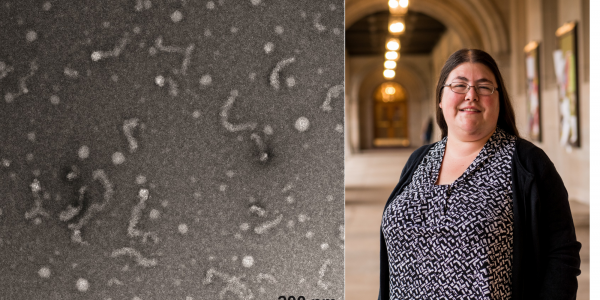
WashU chemists reveal new insights into ALS-linked protein
Using advanced biophysical and imaging techniques, Meredith Jackrel and her team have isolated Matrin-3 to better understand its role in neurodegenerative diseases.

